Scientific Achievement
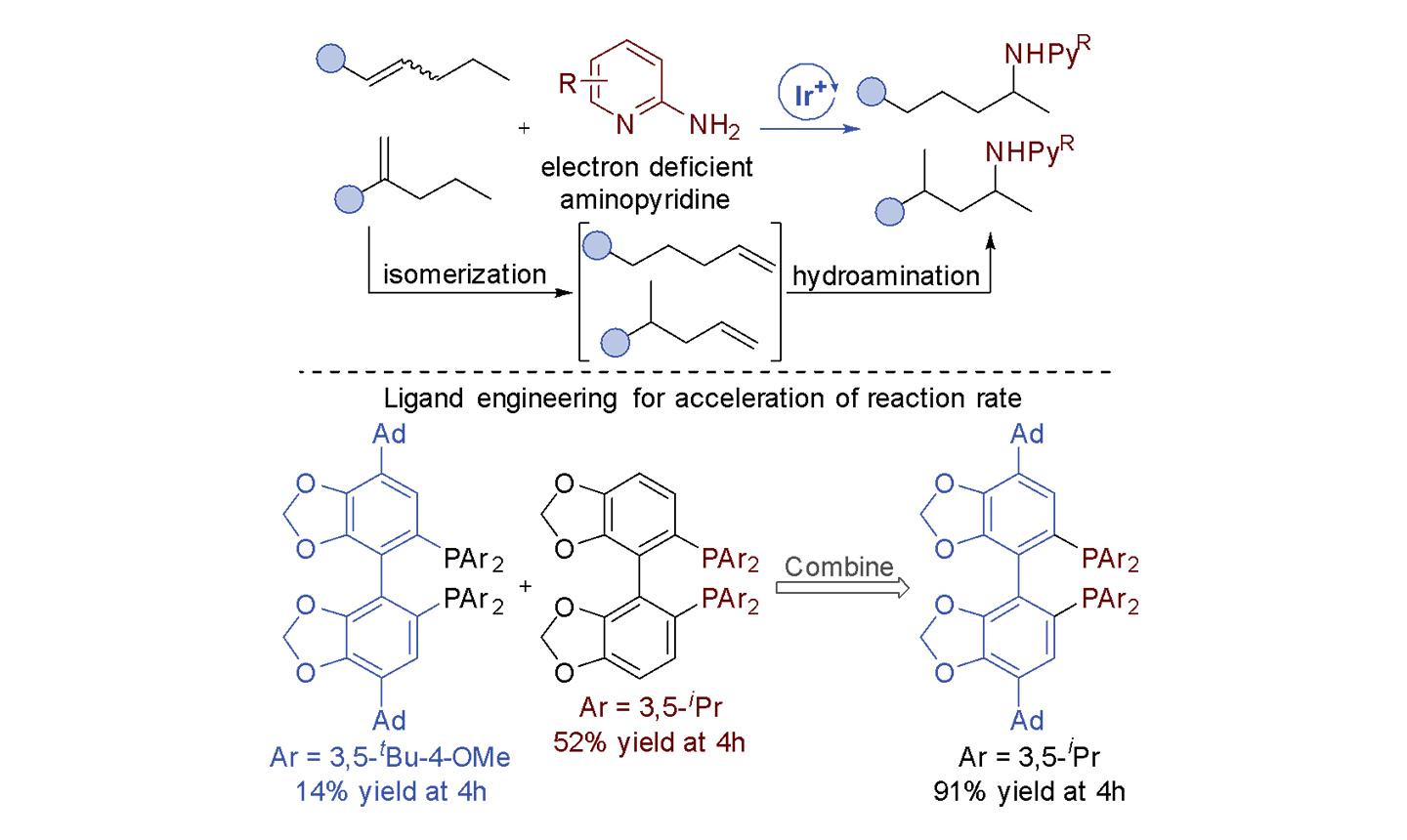
Developed the remote hydroamination of disubstituted alkenes to functionalize an alkyl chain selectively at the subterminal, unactivated, methylene position. The remote hydroamination is compatible with a broad scope of alkenes and aminopyridines and enables the regioconvergent synthesis of amines from an isomeric mixture of alkenes. The products can be derivatized by SNAr with a variety of nucleophiles.
Significance and Impact
This work is a rare example of the addition of the N-H bond of an amine to an alkene in a remote fashion.
Research Details
The use of aminopyridines bearing electron-withdrawing substituents at the 6 position increases the selectivity for the remote amine product. A new DIP-Ad-SEGPHOS ligand with large peripheral groups that positively influence activity and selectivity for the remote hydroamination, likely by non-covalent interactions, was created by evaluating the steric and electronic effects of ligand modules.
Publication Details
Ma, S.; Fan, H.; Day, C. S.; Xi, Y.; Hartwig J. F., J. Am. Chem. Soc. 2023, 145, 3875-3881.
DOI: 10.1021/jacs.2c13054