In an effort to better study a promising class of materials that could energize the solar cell industry, researchers at Lawrence Berkeley National Laboratory (Berkeley Lab) have developed a new method of analyzing the material’s molecular-scale structure.
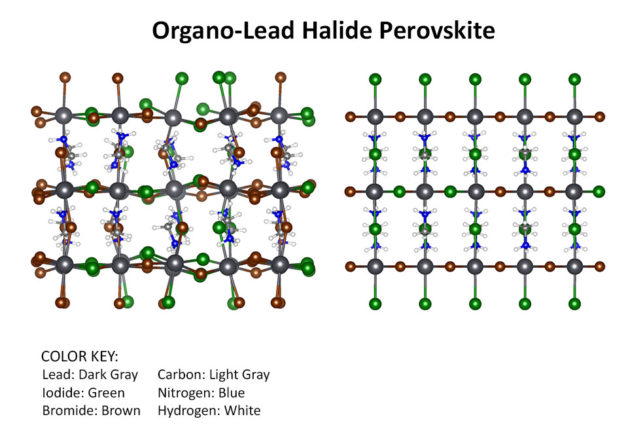
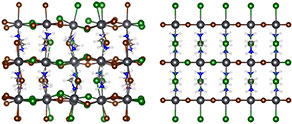
Schematic of organo-lead halide perovskite showing distortions from random halide positions (left) versus ordered halide positions (right). A Berkeley Lab study shows that thermally induced distortions exist in these materials at all iodide/bromide compositions, and that these distortions have a significant impact on the perovskite’s solar cell performance. (Credit: Walter Drisdell/Berkeley Lab)
By combining advanced X-ray spectroscopy measurements with calculations based on fundamental “first principles” theory, researchers obtained an atomic-scale view of organo-lead halide perovskites not easily achieved with current technology.
The approach they are taking works well with structurally disordered materials like halide perovskites, which have garnered intense interest in the solar cell industry because of rapid increases in their photovoltaic efficiency over the past few years. Understanding the structure of perovskites will help researchers determine how to maximize the material’s solar efficiency.
Halides, such as iodide or bromide, are mixed at different ratios to tune properties in the material, like band gaps, that determine solar absorption efficiency. But doing so creates disorder in the structure, making it difficult to use traditional imaging methods.
“Most imaging techniques can’t resolve much of the disordered structure,” said Walter Drisdell, a staff scientist at Berkeley Lab’s Chemical Sciences Division. “X-ray absorption spectroscopy, with high-resolution detection, works because it looks at very local structure and chemical environment around the lead centers without interference from longer-range disorder.”
The researchers used an advanced X-ray spectroscopic technique at the Stanford Synchrotron Radiation Lightsource (SSRL) at DOE’s SLAC National Accelerator Laboratory. They coupled their results with theory work conducted at Berkeley Lab’s Molecular Foundry, where they interpreted the data to understand the structural details of the materials.
“By coupling to our first-principles calculations, we learned that thermal motions, particularly tilts of the lead-halide octahedra, are really important in these materials,” said Drisdell. “The tilts increase the band gap significantly over what we predict for an ordered structure. Before this, little was known about the local structure of these mixed materials, and how that structure affects the large-scale properties that are important for efficient solar devices. We think this work is a milestone that enables significant advances in understanding perovskite photovoltaic materials.”
This work, funded through the Joint Center for Artificial Photosynthesis, sheds light on the chemical structure and dynamics in photovoltaic materials, and could lead to improved designs that maximize solar energy conversion. JCAP is an Energy Innovation Hub supported by DOE’s Office of
Science. The Molecular Foundry and SSRL are DOE Office of Science User Facilities.
More information about this research is available online in the journal ACS Energy Letters.
###
Lawrence Berkeley National Laboratory addresses the world’s most urgent scientific challenges by advancing sustainable energy, protecting human health, creating new materials, and revealing the origin and fate of the universe. Founded in 1931, Berkeley Lab’s scientific expertise has been recognized with 13 Nobel Prizes. The University of California manages Berkeley Lab for the U.S. Department of Energy’s Office of Science. For more, visit www.lbl.gov.
DOE’s Office of Science is the single largest supporter of basic research in the physical sciences in the United States, and is working to address some of the most pressing challenges of our time. For more information, please visit science.energy.gov.